Dye penetration test: Testing the sterile barrier of critical medical devices
Do sterile products remain sterile in your packaging?
We verify this expectation with dye penetration tests performed in accordance with ASTM F1929 (Injection Method, Method A) for porous packaging or ASTM F3039 for non-porous packaging.
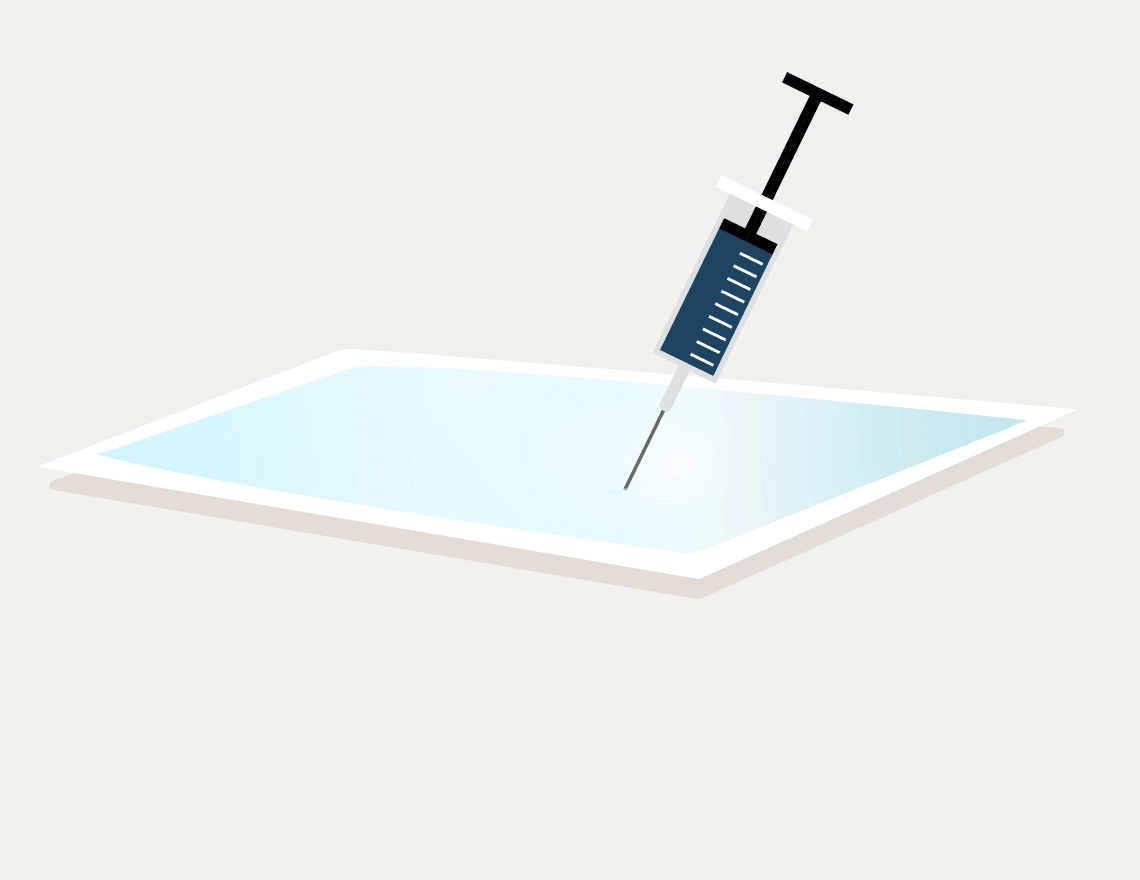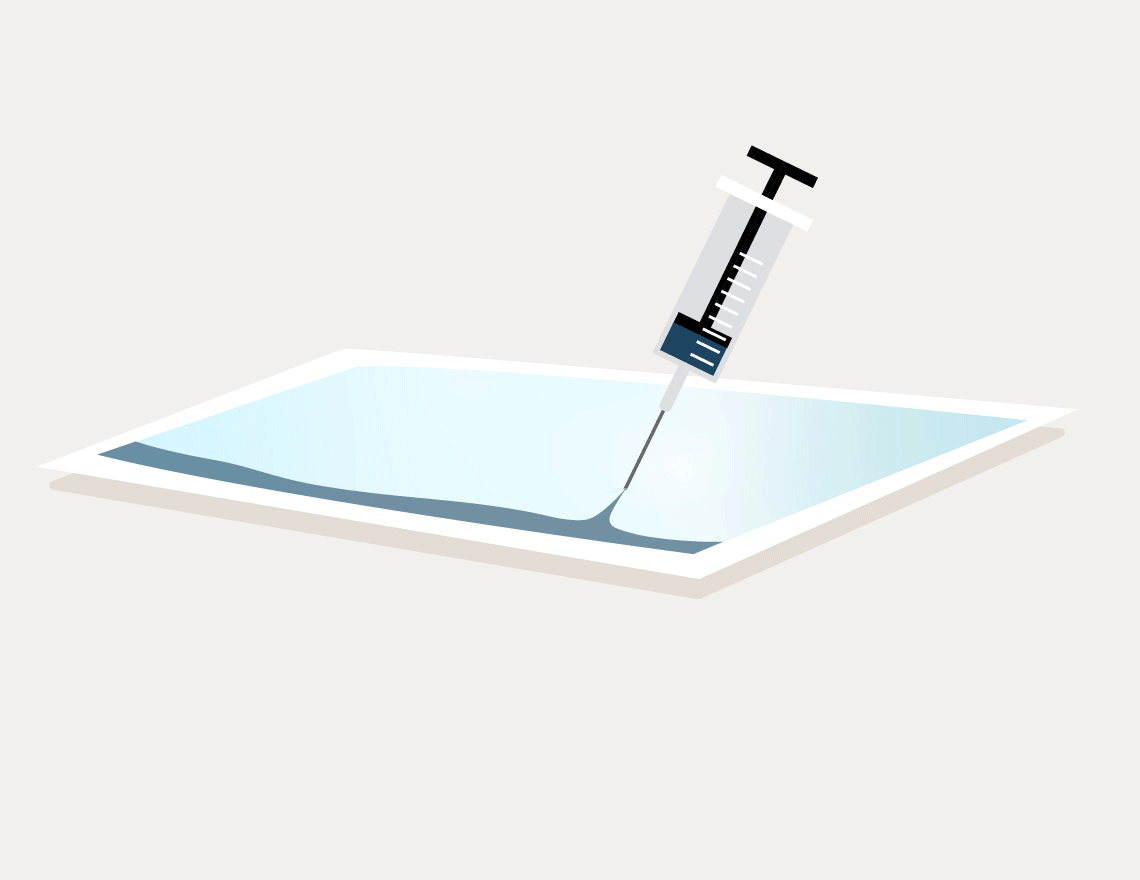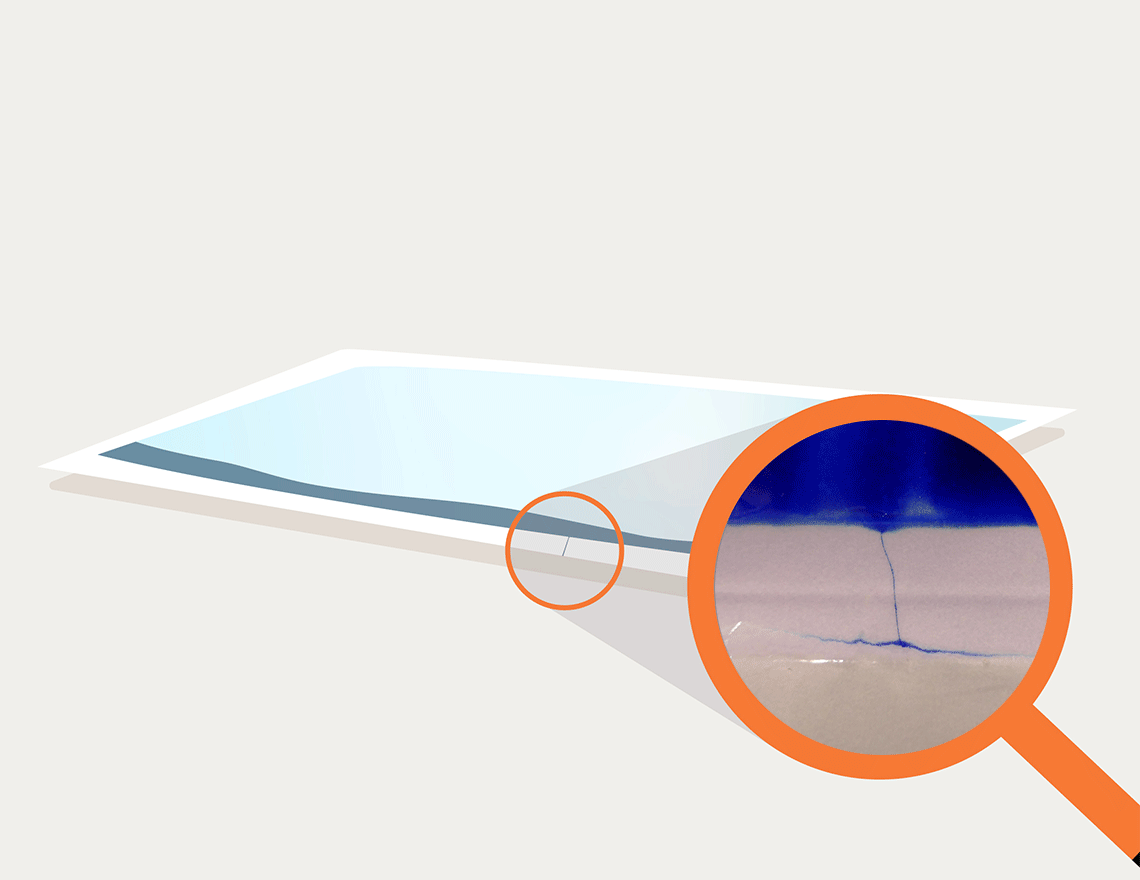
A channel appearing in blue shows the vulnerability
Testing Standards & Equipment
- ASTM F1929
- ASTM F3039
The required samples of critical medical devices do not leave our laboratory and do not have to be subcontracted to other testing services. This gives you the assurance of an inclusive result that automatically combines all the tests performed. The integrity of the sealed seam can be demonstrated without further delay.
Your contacts

Karolina Behrens
Dipl.-Ing.
Head of Laboratory for Shipping Simulation
040 / 5730 944 -27

Johanna Lipski
M. Sc.
Deputy Head of Laboratory for Shipping Simulation
040 / 5730 944 -26
We find answers to your questions
We provide expertise, focus on packaging and find answers to your questions - authentically, honestly, competently and without digressing. So that we can answer your questions, test your products and expand your knowledge of packaging, send us your e-mail address and we will get back to you.
We look forward to your individual challenge!

Our accreditation & certifications

Accreditation
The German Accreditation Body (DAkkS)
is the national accreditation authority
of the Federal Republic of Germany.

Certification

